A prospective, randomized, double-masked, placebo-controlled efficacy and safety study
was conducted in the US. The objective was to evaluate the efficacy and safety of
BlinkTM NutriTears® in adults (N=116) with clinically diagnosed DED. The study found:
BlinkTM NutriTears® Provided
Noticeable, Continuous Symptom Relief
in 2 to 4 weeks.*§
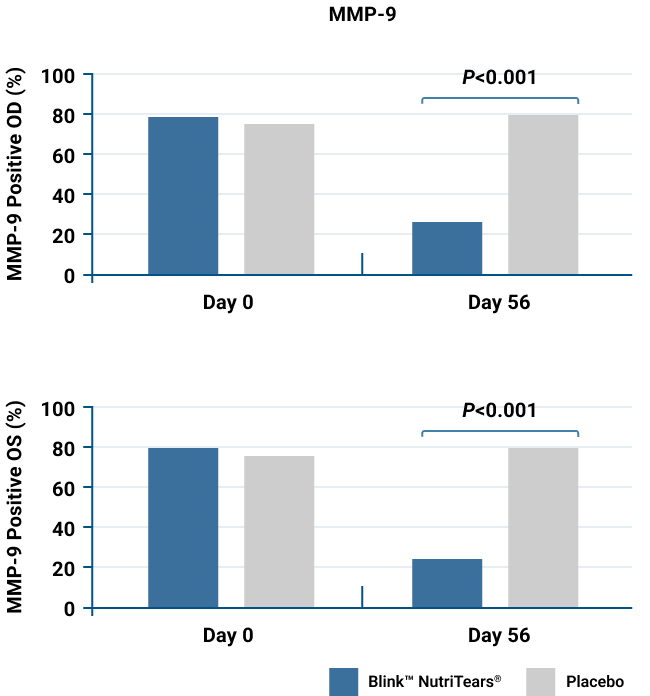
BlinkTM NutriTears® Decreased MMP-9 Levels, Indicating Reduced Inflammation*§
Positive MMP-9 test results decreased from baseline in both eyes for the BlinkTM NutriTears® group and did not decrease for the placebo group.
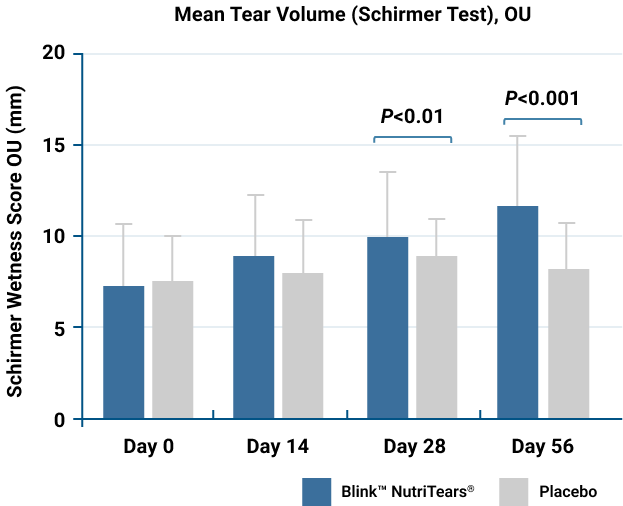
BlinkTM NutriTears® Increased Tear Volume*§
The overall mean tear volume of both eyes was significantly better for the BlinkTM NutriTears® group at Day 56.
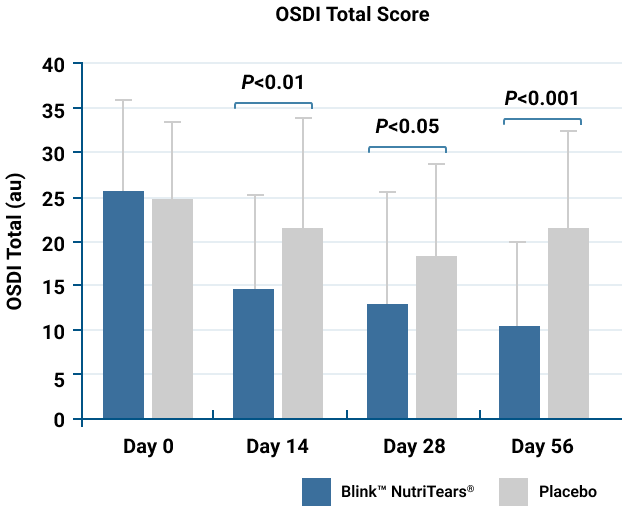
BlinkTM NutriTears® Significantly Reduced the Frequency and Severity of Dry Eye Symptoms in 2 to 4 Weeks*§
Improvement from baseline in total OSDI scores was significantly better for participants in the BlinkTM NutriTears® group.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
§Based on a clinical trial; N=116, randomized 1:1 NutriTears® and placebo.
Discover How BlinkTM NutriTears® Works Differently
Learn More*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
§Based on a clinical trial; N=116, randomized 1:1 NutriTears® and placebo.


*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
§Based on a clinical trial; N=116, randomized 1:1 NutriTears® and placebo.
Frequently Asked Questions
Dive deeper into the clinical data for BlinkTM NutriTears®.
Recent data in patients with DED from India demonstrated that BlinkTM NutriTears® significantly improved tear production, stability, and quality, and reduced inflammation and ocular surface damage in patients with mild-to-moderate DED.
In a similar 8-week study in participants in the US, the BlinkTM NutriTears® group demonstrated significantly better Schirmer’s test scores and improvement in overall OSDI score, versus placebo, at Day 56. Scores for total OSDI, and symptoms and vision domains, significantly improved by Day 14 for BlinkTM NutriTears® versus placebo and were maintained to Day 56. In addition, the BlinkTM NutriTears® group demonstrated significantly improved tear film break-up time (TBUT) and tear film osmolarity versus placebo, along with significant improvements in corneal and conjunctival staining and inflammation.
Clinical studies evaluating BlinkTM NutriTears® versus placebo in patients with dry eye disease were conducted in India (N=59) and the US (N=119). Both studies followed patients for eight weeks. No safety issues were observed in the India study. In the US study, one patient experienced nasal bleeding—a mild adverse event considered possibly related to BlinkTM NutriTears®.
Participants in the BlinkTM NutriTears® clinical studies were followed for eight weeks without safety concerns. Data are not available beyond eight weeks.
In the US study of 155 participants, the BlinkTM NutriTears® group had significant improvement in mean TBUT values, versus the placebo group, in the left eye, right eye, and the mean of both eyes at Day 56. At Day 28, values for the left eye and the mean of both eyes were also significantly improved versus the placebo group.
In the US study of 155 participants, for BlinkTM NutriTears® participants, improvement from baseline in total SPEED score was significantly better (lower scores) by Day 14, both within the LCD group (p<0.001) and versus the placebo group (p<0.05), and this improvement was maintained to Days 28 and 56 (p<0.001 for each timepoint within the LCD group, and p<0.05 and p<0.001, respectively, versus placebo). Scores specifically for the frequency domain mirrored the pattern for total scores and were significantly better for participants in the LCD group at Days 14, 28, and 56, both within the group (p<0.001 for each timepoint) and versus the placebo group (p<0.05, p<0.05, p<0.001, respectively). Scores for the severity domain in the LCD group also decreased from baseline to Day 14, and were significant within the group (p<0.001) at all timepoints; versus placebo, this improvement in severity became significant at Day 28 (p<0.05) and Day 56 (p<0.001).
In the US study of 155 participants, corneal staining and conjunctival staining were significantly improved for the BlinkTM NutriTears® group, versus the placebo group, at Day 56. Mean staining values and osmolarity values for both eyes in the BlinkTM NutriTears® group were significantly lower, versus the placebo group, at Day 56 (p<0.001 for all values). Staining values and osmolarity values specifically in the right eye and left eye were also significantly lower, versus the placebo group, at Day 56 (p<0.01 for staining values, p<0.001 for osmolarity values, respectively).
In the US study of 155 participants, BlinkTM NutriTears® significantly improved (decreased) tear osmolarity by Day 56.


